Central chemoreception is the mechanism by which the brain regulates breathing in response to changes in tissue CO2/H+. A brainstem region called the retrotrapezoid nucleus (RTN) contains a population of CO2/H+-sensitive neurons that appear to function as important chemoreceptors. Evidence also indicates that CO2-evoked ATP release from RTN astrocytes modulates activity of CO2/H+-sensitive neurons; however, the extent to which purinergic signaling contributes to RTN chemoreception is not clear and the mechanism(s) underlying CO2/H+-evoked ATP release is not fully elucidated. To answer these questions at both the cellular and systems level, we use in vitro and in vivo electrophysiological techniques to investigate the contribution of purinergic drive to CO2-sensitivity in various respiratory centers. We are also investigating the possibility that CO2-evoked ATP release influences RTN chemoreceptor activity by regulation of blood flow. It is well established in vivo that changes in cerebral blood flow can profoundly affect the chemoreflex; e.g., limiting blood flow by vasoconstriction acidifies tissue pH and increases the ventilatory response to CO2, whereas vasodilation can wash-out metabolically produced CO2 from tissue to increase tissue pH and decrease the stimulus at chemoreceptors. Determining these mechanisms may lead to new therapeutic avenues for the management of conditions resulting from suppressed respiratory drive.
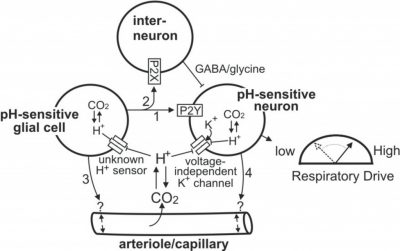
Working Model of Chemoreception by the RTN
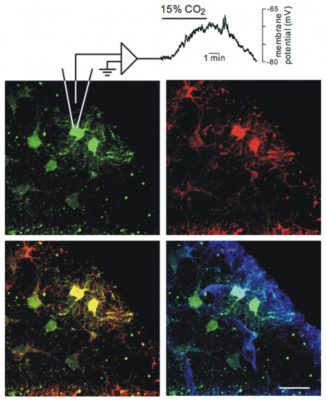
IR-DIC Video of Arteriole Vasomotion in the RTN
The RTN contains a population of CO2/H+-sensitive neurons that appear to function as respiratory chemoreceptors. The RTN also contains a population of pH-sensitive glial cells that may contribute to chemoreception by releasing ATP during hypercapnia. Evidence indicates that ATP can contribute to chemoreception by 1) activating pH-sensitive neurons through a P2Y-receptor-dependent mechanism (arrow 1); 2) inhibiting pH-sensitive neurons by activation of P2X-receptors on interneurons (arrow 2); 3) modulating vascular tone to increase or decrease tissue pH (arrow 3). It is also possible that pH-sensitive neurons influence activity of pH-sensitive glial cells by the release of excitatory neurotransmitters or increased extracellular K+ (arrow 4) or regulate vascular tone directly (arrow 5). Redrawn from J Appl Physiol. 108:1433-9, 2010.